China cosmetics product registration and filing
Legal overview
On January 3, 2020, the Executive meeting of The State Council passed the Regulations on the Supervision and Administration of Cosmetics (Draft) (hereinafter referred to as “ New regulations ”) . On June 29, 2020, the new regulations were officially announced, and will be formally implemented from January 1, 2021, requiring that cosmetics production and operation activities and supervision and management within the territory of the People's Republic of China should comply with the new regulations.
Administrative license approval/filing certificate is an important basis for product import and listing, and cosmetics registration/filing is an indispensable step before its successful sale.
Cosmetics definition
Cosmetics refer to daily chemical industrial products applied to skin, hair, nails, lips and other human surfaces by rubbing, spraying or other similar methods for the purpose of cleaning, protecting, beautifying and modifying.
Cosmetics Declaration Definition
refers to a series of behaviors in which cosmetics enterprises need to submit application materials to relevant departments and complete product filing or approval in order to sell their products in the domestic market. Among them, domestic cosmetics need to be registered in the State Drug Administration, and imported cosmetics need to be registered in the State Drug Administration.
Classification of cosmetics

Chinese cosmetics supervision model
Cosmetics registration requirements
According to the Regulations on the Administration of Cosmetics Registration and Filing, the following materials shall be submitted for the registration and filing of cosmetics:
Cosmetics Registration and Record Information Form and related materials;
Product name information;
Product formula;
Standards of product execution;
Product label sample;
Product inspection report;
Product Safety Evaluation data
Cosmetics label requirements:
According to the "Measures for the Administration of Cosmetics Labels", the Chinese label of cosmetics should include at least the following:
Product name in Chinese, special cosmetics registration certificate number;
The name and address of the registrant or record holder, and if the registrant or record holder is an overseas enterprise, the name and address of the domestic responsible person shall be marked at the same time;
The name and address of the production enterprise, and the production license number of the domestic cosmetics enterprise shall be marked at the same time;
Standard number of product execution;
Whole component;
Net content;
Service life;
Usage method; Necessary safety warning words;
Other contents that should be marked according to laws, administrative regulations and mandatory national standards.
For products with packaging boxes, the Chinese name and service life of the product shall also be marked on the packaging containers that come into direct contact with the contents.
Requirements for cosmetics listing in China:
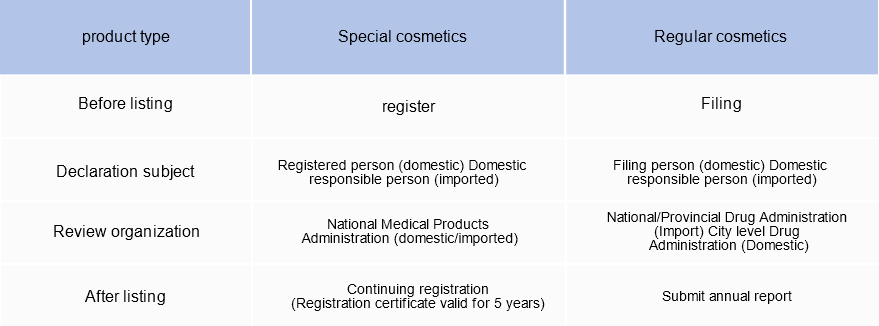
At present, in addition to the cosmetics sold by cross-border e-commerce channels are exempt from registration and filing, cosmetics sold by other channels (except ordinary beauty soap) need to be registered and filed by the National Drug Administration (NMPA) or the local drug administration before they can be sold. The state shall exercise registration administration for special cosmetics and archival administration for ordinary cosmetics.
Cosmetics registrants, record requirements:
The registrant and record holder of cosmetics shall be responsible for the claim of quality safety and efficacy of cosmetics. It must meet the following conditions: It is an enterprise or other organization established according to law; There is a quality management system suitable for the products applied for registration and filed; Ability to monitor and evaluate adverse reactions of cosmetics.
Domestic liability:
Overseas cosmetics registrants and recordholders shall designate domestic enterprise legal persons to handle cosmetics registration and recordkeeping, assist in the monitoring of cosmetic adverse reactions, and implement product recalls. The domestic responsible person shall perform the following obligations:
Handle the registration and filing of cosmetics and cosmetics new raw materials in the name of the registrant and record holder;
Assist registrants and record holders to carry out adverse reaction monitoring of cosmetics, safety monitoring and reporting of new raw materials of cosmetics;
Assist registrants and record holders to implement the recall of cosmetics and cosmetics new raw materials;
Undertake corresponding quality and safety responsibilities for cosmetics and new raw materials put on the domestic market in accordance with the agreement with the registrant and the record holder;
Cooperate with the supervision and inspection work of the drug regulatory department.

The services we provide
- • China Cosmetics product registration and filing
- • China Cosmetics formula label review
- • Safety Assessment of cosmetics in China
- • Efficacy evaluation of Chinese cosmetics
Zhongbang is one of the earliest institutions in China to engage in compliance consulting services. It has a senior expert team composed of PhDs, Masters, and Bachelor's degrees, and has established branches in the UK, the United States, South Korea, Beijing, Shanghai, Suzhou, Fuzhou, Shenzhen, and other places, forming a global service network layout.
-
Strong technical strength:
A strong team composed of mid to senior technical personnel with professional backgrounds in chemistry, food nutrition and health, medicine, biology, toxicology, environment, and other fields
-
International service level:
A business elite team with multiple languages including English, Japanese, German, and Chinese, efficiently serving international customers
-
Rich compliance experience:
With years of experience in global product and regulatory research in multiple fields, we are able to provide comprehensive and high-level compliance consulting support to various customers
-
Localized service capabilities:
Having multiple branch offices in the UK, USA, South Korea, Beijing, Shanghai, Suzhou, Fuzhou, Shenzhen, etc., we can provide you with services nearby
Service Hotline :400-115-9001
Zhongbang Consulting (Shandong Zhongbang North Management Consulting Co., Ltd.) focuses on product registration and compliance consulting services. With its professional technology, diverse resources, and global network, we are committed to providing regulatory consulting and environmental regulation response services for pharmaceutical, chemical, consumer goods production enterprises, and large multinational corporations. To solve the environmental, health and safety laws and regulations, product quality standards, and other issues faced by enterprises in production, sales, and global trade processes.
Accumulated Customers
18,000+
Distributed in 35 countries and regions
14year
Industry experience
260+
Senior expert team
15+
Subsidiaries
300+
Collaboration Cases
Brand Cases
The choices and trust of over 18000 customers are the driving force for Zhongbang to continuously improve its services. We are willing to grow together with these excellent enterprises
Service Hotline:400-115-9001







































































 Consultation
Consultation
 400-115-9001
400-115-9001