China cosmetics new raw materials filing/registration
Legal overview
As the cosmetics industry enters “ Age of efficacy ” Consumers' awareness of cosmetic ingredients and efficacy continues to improve; The Regulation on Supervision and Administration of Cosmetics is clear “ Encourage and support the use of modern science and technology, combined with China's traditional advantages of projects and characteristic plant resources research and development of cosmetics ” . With the support of both market and regulatory levels, many companies are beginning to explore and verify the efficacy of Chinese ingredients, “ Chinese ingredients ” It has become one of the hottest words in the cosmetics industry in recent years.
Details of cosmetic new raw materials registration and filing project
(A) raw material research and development background, including research and development background, research and development purpose, research and development process and research and development results;
(2) basic information of raw materials, including the name, source, composition, relative molecular weight, molecular formula, chemical structure, physical and chemical properties of raw materials;
(3) Information on the use of raw materials in cosmetics, including the specifications, purpose of use, application or scope of use, safe use, use period, precautions, warning words, etc.; The status and approval status of the raw materials used in cosmetics abroad;
(4) Functional basis data, the functional basis of new cosmetic raw materials refers to the relevant data that can prove that the raw materials are consistent with the purpose of use, generally including scientific literature, regulatory data, laboratory research data, and human efficacy evaluation test data;
(5) Other information related to the development of new raw materials.
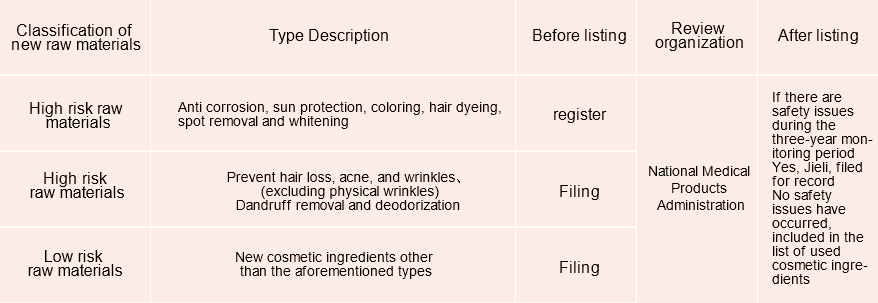
Supervision mode of new raw materials of cosmetics in China
According to the Regulations on the Supervision and Administration of Cosmetics, the state implements registration management for new raw materials of cosmetics with a high degree of risk (referring to new raw materials of cosmetics with anti-corrosion, sun protection, coloring, hair coloring, freckle whitening function), and implements filing management for other new raw materials of cosmetics.
According to the "Measures for the Administration of Cosmetics Registration and Filing", the new cosmetic raw materials that have been registered and completed the filing shall implement the safety monitoring system, and the safety monitoring period shall be 3 years (from the date on which the cosmetics that have used the new raw materials for the first time obtain registration or complete the filing). During the period of safety monitoring, the registrant and recordholder of new raw materials shall report the use and safety of new raw materials to the drug regulatory department under The State Council annually. For new cosmetic raw materials with safety problems, the drug regulatory department under The State Council shall cancel the registration or cancel the record. New cosmetic raw materials that have not caused safety problems within three years shall be included in the catalogue of used cosmetic raw materials formulated by the drug regulatory department under The State Council.
New material registration & User information to be submitted by the filer
1, cosmetic new raw materials registrant, record person information;
2. Overview of safety risk monitoring and evaluation system for registrants and recordholders of new cosmetic raw materials;
3, cosmetics new raw materials registrant, record is overseas, shall be filled in by the domestic responsible person information, and submit the original letter of authorization and notarial certificate of the domestic responsible person.
New material registration & File submissions
1. Name, address and contact information of the registrant, the record holder and the domestic responsible person;
2. New raw material development report;
3. Research data on the preparation process, stability and quality control standards of new raw materials;
4. Safety assessment data of new raw materials.
Registration cycle of new raw materials
Test cycle: It takes 2 months to 4 years to test toxicological items and product efficacy according to different circumstances of the product, and the stability test time under different shelf life needs to be considered.
Registration period: 5-9 months; Filing period: 1-2 months.
The services we provide
- ○ Cosmetic raw material quality and safety information reporting service
- ○ Test supervision services for new cosmetic raw materials such as physicochemistry/Toxicology/efficacy
- ○ Safety Assessment Service for new cosmetic ingredients
- ○ Cosmetics new raw materials filing/registration process tracking
Zhongbang is one of the earliest institutions in China to engage in compliance consulting services. It has a senior expert team composed of PhDs, Masters, and Bachelor's degrees, and has established branches in the UK, the United States, South Korea, Beijing, Shanghai, Suzhou, Fuzhou, Shenzhen, and other places, forming a global service network layout.
-
Strong technical strength:
A strong team composed of mid to senior technical personnel with professional backgrounds in chemistry, food nutrition and health, medicine, biology, toxicology, environment, and other fields
-
International service level:
A business elite team with multiple languages including English, Japanese, German, and Chinese, efficiently serving international customers
-
Rich compliance experience:
With years of experience in global product and regulatory research in multiple fields, we are able to provide comprehensive and high-level compliance consulting support to various customers
-
Localized service capabilities:
Having multiple branch offices in the UK, USA, South Korea, Beijing, Shanghai, Suzhou, Fuzhou, Shenzhen, etc., we can provide you with services nearby
Service Hotline :400-115-9001
Zhongbang Consulting (Shandong Zhongbang North Management Consulting Co., Ltd.) focuses on product registration and compliance consulting services. With its professional technology, diverse resources, and global network, we are committed to providing regulatory consulting and environmental regulation response services for pharmaceutical, chemical, consumer goods production enterprises, and large multinational corporations. To solve the environmental, health and safety laws and regulations, product quality standards, and other issues faced by enterprises in production, sales, and global trade processes.
Accumulated Customers
18,000+
Distributed in 35 countries and regions
14year
Industry experience
260+
Senior expert team
15+
Subsidiaries
300+
Collaboration Cases
Brand Cases
The choices and trust of over 18000 customers are the driving force for Zhongbang to continuously improve its services. We are willing to grow together with these excellent enterprises
Service Hotline:400-115-9001







































































 Consultation
Consultation
 400-115-9001
400-115-9001