Korea publishes guidelines on the joint registration process for existing substances under K-REACH
In order to promote the progress of the implementation of K-REACH joint registration, the Korean Ministry of Environmental Protection officially released the Korean and English versions of the K-REACH joint registration process guidance document for existing substances on September 22. Sino-state Consulting hereby collates the key contents of the guide
In order to promote the progress of the implementation of K-REACH joint registration, the Korean Ministry of Environmental Protection officially released the Korean and English versions of the K-REACH joint registration process guidance document for existing substances on September 22. Sino-state Consulting hereby collates the key contents of the guide document that are closely related to enterprises to help enterprises better cope with K-REACH.
K-REACH requirements fall into the first batch list and are manufactured or imported ≥ 1 ton/year of existing substances must be jointly registered, and the registration buffer period is three years. Rio summarizes the main steps in the guidance document for joint submission as follows:
- Identify potential registrants
- Lead Registrant Selected (LR)
- Survey available data
- Analysis data gap
- Purchase or generate other required data
- Cost sharing
- Prepare and submit files
Companies can find potential registrants of each existing substance through the Joint Registration Information System, but all potential registrants need to fill in the following information before they can join the system:
- Company name
- Business Registration Number
- Contact us
- Tonnage
- Production/Import information
- Proof of production/import of any existing substance
- Whether the registrant intends to be the lead registrant
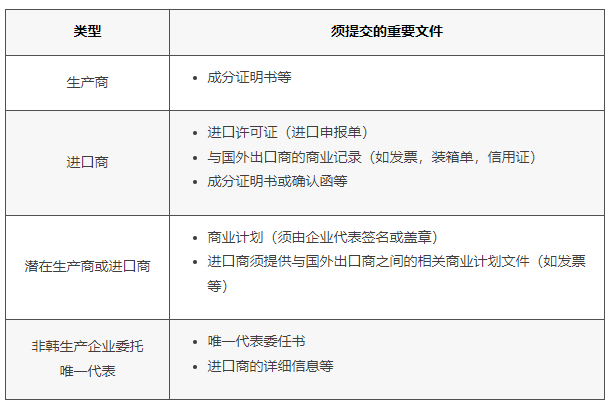
At the same time, different enterprises importing/producing existing substances must submit the corresponding supporting documents, which are specified as follows:
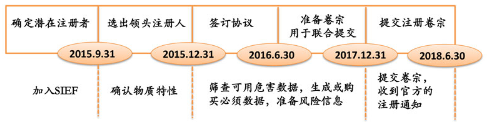
The following is the official recommended schedule for the joint registration of existing substances, and enterprises can also adjust the registration time and strategy according to the actual situation.
Because the joint registration of chemical substances needs to go through many complex processes from the formation of a registration consortium, the acquisition of data and information, the development of experiments to the production of dossiers, and the official implementation of K-REACH joint registration for the first time, the three-year buffer period is not sufficient. Riou recommends that companies join the system in a timely manner to form a consortium and ensure that joint registration is completed before the deadline.



 Consultation
Consultation
 400-115-9001
400-115-9001