Introduction of radiofrequency beauty instrument and design of clinical trial
Radiofrequency beauty instrument (also known as radiofrequency therapy instrument or radiofrequency skin therapy instrument) refers to the use of a specific frequency of radiofrequency current (usually about 200k-5MHz) or electric field (usually 13.56 or 40.68MHz) and other electrical energy acting on human tissues to produce thermal effects. Products
Radiofrequency beauty instrument (also known as radiofrequency therapy instrument or radiofrequency skin therapy instrument) refers to the use of a specific frequency of radiofrequency current (usually about 200k-5MHz) or electric field (usually 13.56 or 40.68MHz) and other electrical energy acting on human tissues to produce thermal effects. Products designed to treat sagging skin, reduce skin wrinkles, shrink pores, tighten/lift skin tissue, or treat acne, scarring, or reduce fat (lipopalacia or breakdown).
The early RF beauty instrument is relatively large, the price is very expensive, and the RF head is easy to lose, the output energy level of the instrument is high, and the damage to the epidermis is also large, so it is mostly used for medical treatment, and can only be used in the hospital, and the delay period after the patient receives treatment is longer. Now, beauty instrument manufacturers have produced small radiofrequency instruments that can be used for home use, which have no impact on the appearance of the skin after treatment (there is no wrong duration), and the cost of use is greatly reduced, so it is very popular.
On March 24, 2022, the State Food and Drug Administration dynamically adjusted the 10 categories of medical device products, clarified that the RF beauty instrument was the third category of medical device management, and all RF beauty instrument products required to obtain the medical device registration certificate from April 1, 2024 before production and sales.
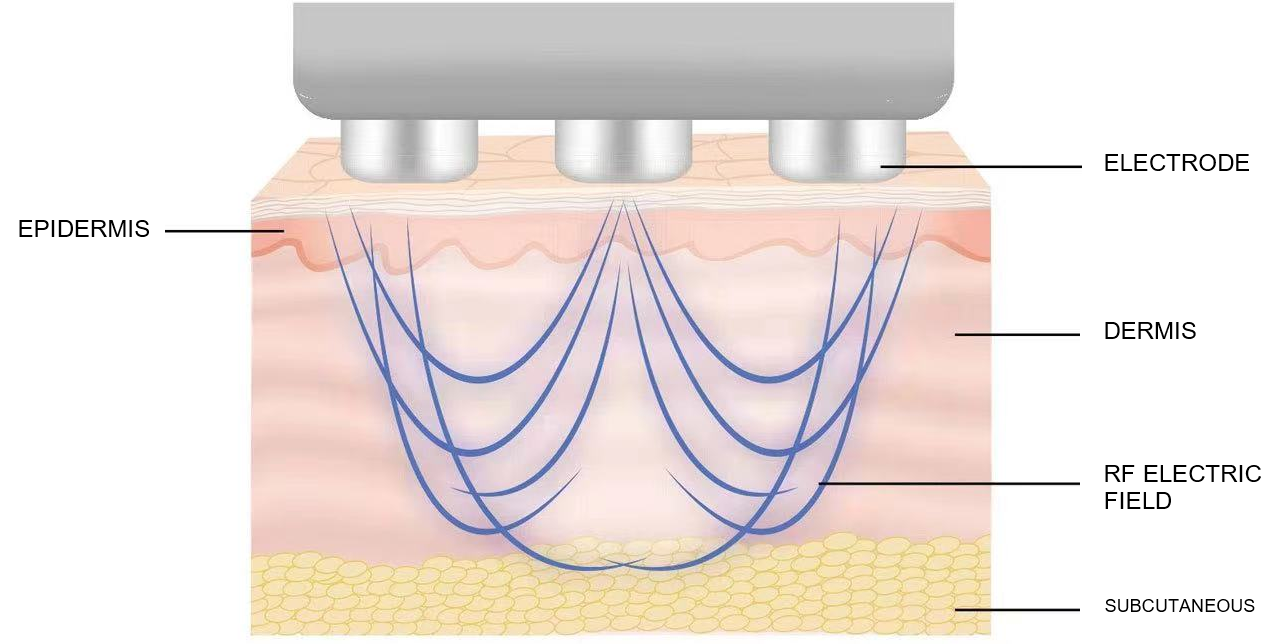
RF beauty instrument needs to evaluate product safety and effectiveness through clinical trials in the product registration process. The following clinical trial design ideas for RF beauty instrument are proposed.
Design of clinical trial scheme of RF beauty instrument
Protocol design: Prospective, multicenter, randomized, parallel-controlled, evaluator blind, non-inferior clinical trial.
Inclusion criteria mainly include:
(1) Age & ge; Healthy adults of 20 years of age, regardless of gender;
(2) Subjects who have different degrees of aging on their facial skin and need facial treatment;
(3) Voluntarily participate in this trial, be willing to complete follow-up, and sign a written informed consent.
exclusion criteria mainly include:
(1) Subjects who have received pacemakers, stents, or metal implants;
(2) Subjects with a history of severe skin allergy;
(3) Subjects with a history of skin diseases, keloids, abnormal wound healing, and sensitive dry skin or cicatricial constitutions;
(4) Subjects with facial nerve injury.
Clinical evaluation indicators of RF cosmetic instrument:
1. Main measure: wrinkle improvement rate (facial Fitzpatrick Wrinkle and elasticity Rating Scale).
2. Secondary evaluation indicators: GASI (global aesthetic improvement scale) score, Visual Analogue Scale (VAS) pain score, VISIA detection, etc.
3. Safety evaluation index:
(1) Vital signs;
(2) Adverse events (mostly temporary) : mainly included headache, transient erythema, edema, second-degree burn, transient skin pitting, fat necrosis and fat atrophy, transient pigmentation, subcutaneous nodules, hematoma, skin numbness in the treated area, scar, etc.




 Consultation
Consultation
 400-115-9001
400-115-9001