2025 Pharmacopoeia "Metal components and containers for Drug packaging" general rules come
On August 15, 2023, the official website of the National Pharmacopoeia Committee issued the "Letter on soliciting opinions on the 9 general Rules of Metal drug Packaging Materials of the Chinese Pharmacopoeia", which intends to solicit opinions on the 5400 "Metal components and containers for Drug Packaging" of the 2025 edition of the Chinese Pharmacop
On August 15, 2023, the official website of the National Pharmacopoeia Committee was published “ Letter on soliciting opinions on 9 General Rules of Metal Drug Packaging Materials in Chinese Pharmacopoeia ” It is proposed to solicit opinions on the 5400 "Metal Components and Containers for Drug Packaging" that will increase revenue in the 2025 edition of the "Chinese Pharmacopoeia", and the publicity period will be 1 month from the date of release. This is according to the overall planning and preparation of the Chinese Pharmacopoeia 2025 version of the pharmaceutical packaging material standard system. This general rule specifies the basic requirements that should be met in the development, production, use and quality control of metal components and containers for drug packaging systems. Zhongbang Consulting has sorted out the key points of 5400 "Metal Components and containers for drug Packaging" for your reference.

First, understand pharmaceutical packaging materials — — Metal packaging related terms
1. Pharmaceutical packaging materials
Pharmaceutical packaging material refers to the packaging materials and containers in direct contact with the drug, which can protect the drug from environmental effects and maintain the original properties of the drug. Pharmaceutical packaging material is an important part of pharmaceutical preparation, and qualified pharmaceutical packaging material is the premise to ensure the efficacy of pharmaceutical preparation. According to the classification of material composition, the common pharmaceutical packaging materials mainly have six categories of packaging: plastic packaging, glass packaging, rubber packaging, metal packaging, pre-potting packaging and other packaging.
2. Metal materials for drug packaging
Under normal conditions of use, materials of various metals (including various metal coatings and alloys and metals used in combination) that are intended or have been used in pharmaceutical packaging.
3. Metal components for drug packaging
Under normal conditions of use, a certain form of metal packaging component made of various metal (including various metal coatings and alloys) materials that are expected or have been applied to pharmaceutical packaging by forging, drawing, calendering, etc.
4. Metal containers for drug packaging
Containers made of various metal (including various metal coatings and alloys) materials intended or already used in pharmaceutical packaging under normal conditions of use.
Metal components and containers should be in accordance with the requirements of quality risk control, select the appropriate quality requirements of the project, develop product standards or quality agreements, and formulate inspection rules according to the production and use of risk management requirements. In the process of production and use, appropriate items under the Category General Rules shall be selected for inspection according to the needs of specific preparations, including but not limited to the provisions of these General Rules. For inspection items directly or indirectly related to safety, limit indicators should generally be used to control the quality control items as follows:
1. Identify
The material composition of the metal substrate and coating used should be consistent with the corresponding composition of the product defined or labeled composition and grade, according to the inspection frequency confirmed by the supply and demand parties, the metal substrate should be in accordance with the metal material element content determination method (General rule 4250), and the composition and/or impurity content should meet the relevant provisions of the enterprise standard or quality agreement. Through the limitation requirements of raw material composition, the responsibilities and obligations of raw material production suppliers in the supply chain are clarified, including the responsibilities and obligations of raw material quality control and information transmission.
2.Physical and chemical properties
For the key quality attributes of metal materials, according to the risk degree of the dosage form combined with product characteristics, establish the relevant limit indicators. Packaging of metal containers: Take an appropriate amount of the complete sample and fill it with extracting medium water, 4% acetic acid and 50% ethanol to the labeled capacity of 70℃± according to the determination method of dissolved substance of pharmaceutical packaging materials (general rule 4204); It was kept at 2℃ for 24h and filled to the indicated capacity of 58℃± with the extraction medium n-hexane. After being kept at 2℃ for 24h, the preparation of metal components and container dissolvates for pharmaceutical packaging is shown in Table 1.
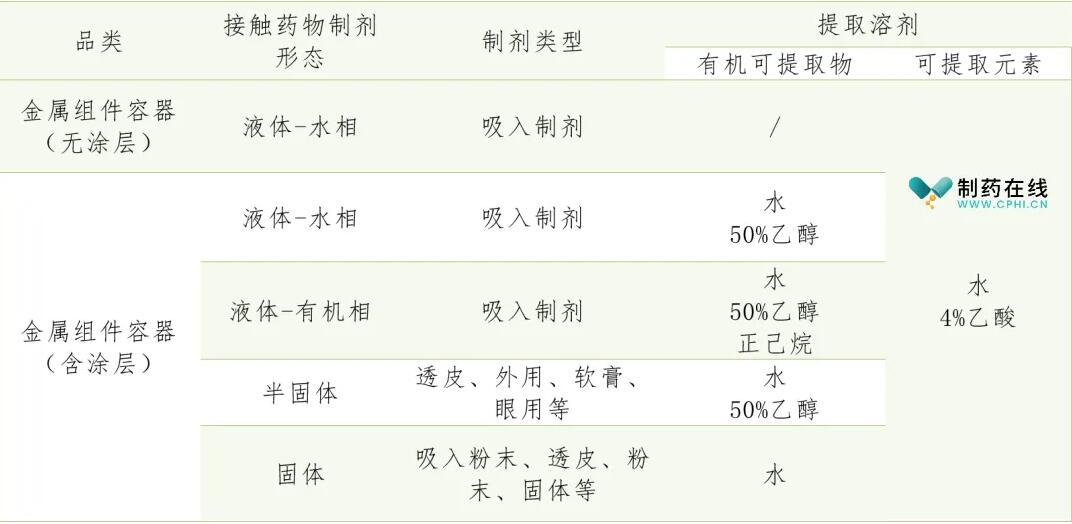
The following items were determined according to the dissolution determination method of pharmaceutical packaging materials (General rule 4204).
2.1 Easy oxide
The sample solution is prepared according to Table 1, and the determination of the dissolved substance of the packaging material of the water extraction solution (General Rule 4204) shall comply with the provisions of Table 2.
2.2 Non-volatile matter;
Prepare the sample solution according to Table 1, and take the organic extractable solution according to the dissolution determination method of the pharmaceutical packaging material (General Rule 4204), which shall comply with the provisions of Table 2.
2.3 Total Organic Carbon (TOC)
The sample solution was prepared according to Table 1, and the water extract solution was determined according to the dissolution method of pharmaceutical packaging materials (General rule 4204) and the total organic carbon determination method in pharmaceutical water (general rule 0682).
2.4 Metal ions
Based on the current status of the application of ICH Q3D requirements for the quality control of harmful elements in drugs in China, add metal ion inspection items, pay attention to the formula of metal materials and types of harmful elements, and establish the scope of concern and investigation of elements according to class 1, Class 2A, class 3 and other elements. Attention is paid to the types of harmful elements in the formulation and process of metal materials. The inspection of elemental impurities refers to the ICH Q3D Guidelines on elemental impurities to provide guidance for the safety assessment of extraction of elemental impurities by inhalation, parenteral, dermal, percutaneous, and oral administration. The sources of known or potential elemental impurities are identified, and the risk assessment of impurity elements is conducted in accordance with drug quality requirements. Because the formulation composition of metal components and containers used in pharmaceutical packaging is known, the ICH Q3D Guidelines for Elemental Impurities establish permissible daily exposures (PDE) for each element of toxicological concern and adopt a risk-based approach to controlling elemental impurities in pharmaceutical products.
Acceptance criteria: Select the appropriate soaking medium according to Table 1 for the preparation simulation of metal components and dissolved substances in containers for drug packaging in direct contact with drugs, and conduct element assessment with reference to Table 3. For the quantitative toxicological safety risk assessment of extractable elements consistent with ICH Q3D procedures, use ICH Q3D PDE values as appropriate. The reference 30% threshold is used to determine whether a means of controlling a particular extractable element is needed. At the same time, refer to Table 3 to focus on other types of elements in the materials and establish an assessment method in combination with the form of pharmaceutical preparations to assess the impact of packaging materials.
2.5 Coating monomer migration
For metal components and packaging using organic coatings, it is necessary to pay attention to the migration of coating monomers (including but not limited to bisphenol A, terephthalic acid, acrylic acid, caprolactam, butanediol, formaldehyde, etc. *) according to Table 1 for the preparation of metal components and containers for drug packaging in direct contact with drugs, and set up limit indicators based on the degree of risk of the dosage form. It shall comply with the provisions of the category general rules, enterprise standards or quality agreements.
Remarks * Including but not limited to the above coating monomers, the manufacturer and user of the pharmaceutical packaging material should develop appropriate coating monomer standards and indicators combined with the type of coating coating used, and develop related protocols.
2.6 Inner coating continuity
For metal containers with inner coatings and coatings, check in accordance with the continuous determination method for metal inner coatings (general 4058), and the determination current shall not exceed 40mA.
2.7 Corrosion resistance
Where applicable, metal components and containers in direct contact with the drug product shall be inspected in accordance with the determination of the corrosion resistance of metals (General Rule 4050), and no obvious corrosion shall be observed.
3. Other requirements. — Sterility, microbial limits, or bioloaded
According to the actual situation, select the corresponding sterility, microbial limit or biological load according to different uses and production and use methods for inspection.





 Consultation
Consultation
 400-115-9001
400-115-9001