paramylon: A new food for the EU?
On May 26, 2023, according to the European Food Safety Authority (EFSA), the EU Nutrition, Novel Foods and Food Allergens (NDA) Study Group (hereinafter referred to as the "Group") issued a scientific opinion on the safety of paramylon as a Novel Food.

On May 26, 2023, according to the European Food Safety Authority (EFSA), the European Union Research Group on Nutrition, Novel Foods and Food Allergens (NDA) (hereinafter referred to as “ The group ”) Scientific opinion on the safety of paramylon as a Novel Food.
After an evaluation, the panel concluded that paramylon is safe as a novel food under the proposed conditions of use.
1.Origin and usage history of NF
paramylon (hereinafter referred to as ” The NF”) A linear, unbranched &beta isolated from single-celled microalgae Euglena gracilis; -1, 3-D-glucan polymer.
The source of
NF, E. gracilis, has a history of use as a food (food ingredient and food supplement) in China, Japan, and the United States. In 2020, dried E. gracilis is authorized for sale on the EU market. Authorized uses include as a food supplement and as a food ingredient added to some foods.
In the United States, NF has been available as a food supplement and food ingredient since 2019. In 2021, the Brazilian Health Regulatory Agency (ANVISA) authorized the placing of the NF on the market.
2.NF production process
The NF was obtained by E. gracilis after fermentation, separation, purification, drying and other processing steps.
In 2019, E. gracilis has been recommended to the QPS list for use “ For production purposes only ” Including food products based on microalgal biomass. In this NF production process, E. gracilis is inactivated by the production process.
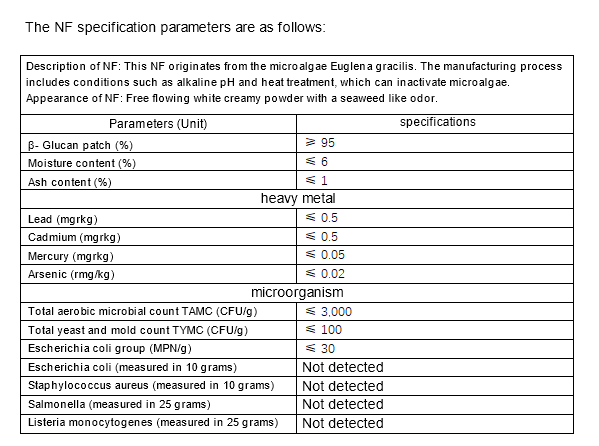
3.NF composition and specifications
The applicant has provided analytical data for five batches of the NF component, showing that the NF is composed of at least 95% dietary fiber (β Beta-glucan) and small amounts of protein, fat, ash and water.
4. Proposed conditions of use of NF
The Applicant proposes to use the NF as a food supplement, for ingredients added to a variety of common foods, and for weight control meal replacement foods.
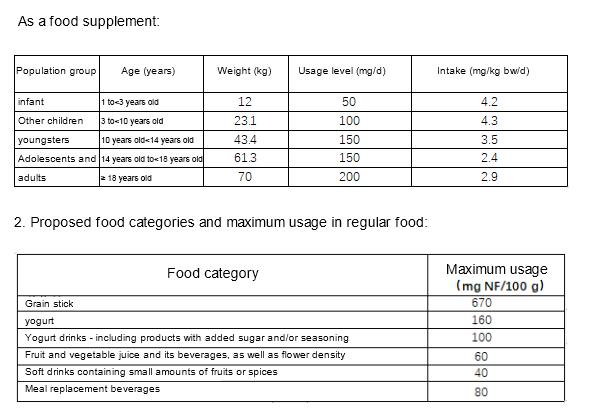
3. For weight control meal replacement food:
The maximum recommended dosage is 600 mg NF/d, and the proposed target population is adults.
5.NF related test and analysis
Heavy Metals, Microorganisms and Contaminants
The applicant provided five batches of testing data for heavy metals (lead, cadmium, mercury, arsenic) and microorganisms (aerobic microorganisms, coliforms, yeasts and molds, Escherichia coli, Staphylococcus aureus, Salmonella, Listeria monocytogenes) of this NF.
The applicant also provided analytical reports of five batches of polycyclic aromatic hydrocarbons (PAHs) and aflatoxin B1, B2, G1 and G2 for NF, with the former below the detection limits (< 3. g/kg), the latter are also below the detection limit (< 3. g/kg).
The Panel finds that the information provided by the applicant about the ingredients is sufficient and does not raise safety concerns.
Stability
The applicant submitted two accelerated stability studies (40° C, 75% relative humidity (RH)) and one environmental condition (25° Long-term (4 years) stability studies of C and 60%RH). In addition, NF was prepared into capsules (mixed with microcrystalline cellulose) under normal environmental conditions (25 ° C, 60% relative humidity) to evaluate its stability for up to 24 months. Finally, the applicant also tested the stability of the NF at various pH values.
The team believes that these data provide sufficient information about the stability of NF.
Absorption, Distribution, Metabolism and excretion (ADME)
The applicant has not submitted an ADME study on this NF. The applicant indicates that the NF consists of at least 95% insolubility β Beta-glucan is composed and passes through the human gastrointestinal tract without digestion, so specific ADME studies are not required.
The panel agreed with the explanation given by the applicant.
Nutritional information
The NF consists of at least 95% β -1, 3-glucan composition. The applicant provided analysis of NF analogues and some minerals at concentrations that did not raise safety concerns
The Panel found that, given the composition of NF and the proposed conditions of use, consumption of NF would have no adverse nutritional effects.
Toxicology
The applicant provided four toxicological studies of this NF, namely in vivo micronucleus test, 14-day oral toxicity/palatability studies and two 90-day oral toxicity studies. In addition, the applicant also submitted the dry matter of Gymnococcus gracilis (58.8% β One acute oral toxicity study and two genotoxicity studies of beta-glucan.
No safety concerns were raised by this NF in the toxicity studies submitted.
Human studies
The applicant does not provide this content.
Allergic
According to the batch test provided by the applicant, NF contains a protein concentration of 0.4-1.1%.
The applicant conducted a comprehensive literature search and found no studies or case reports indicating potential anaphylaxis of NF. In addition, the source of this NF, E. gracilis, has a history of use in China, Japan, and the United States, and no allergic reactions have been reported.
The team concluded that the risk of allergic reaction to this NF in the general population is unknown, but is expected to be low.
6. The panel's assessment of the NF
After an evaluation, the panel concluded that paramylon is safe as a novel food under the proposed conditions of use.






 Consultation
Consultation
 400-115-9001
400-115-9001