The U.S. Food and Drug Administration (FDA) has issued 11 substances through the U.S. Food Contact Materials Notice (FCN), with the following details:
The U.S. Food and Drug Administration (FDA) has issued 11 substances through the U.S. Food Contact Materials Notice (FCN) with the following details:
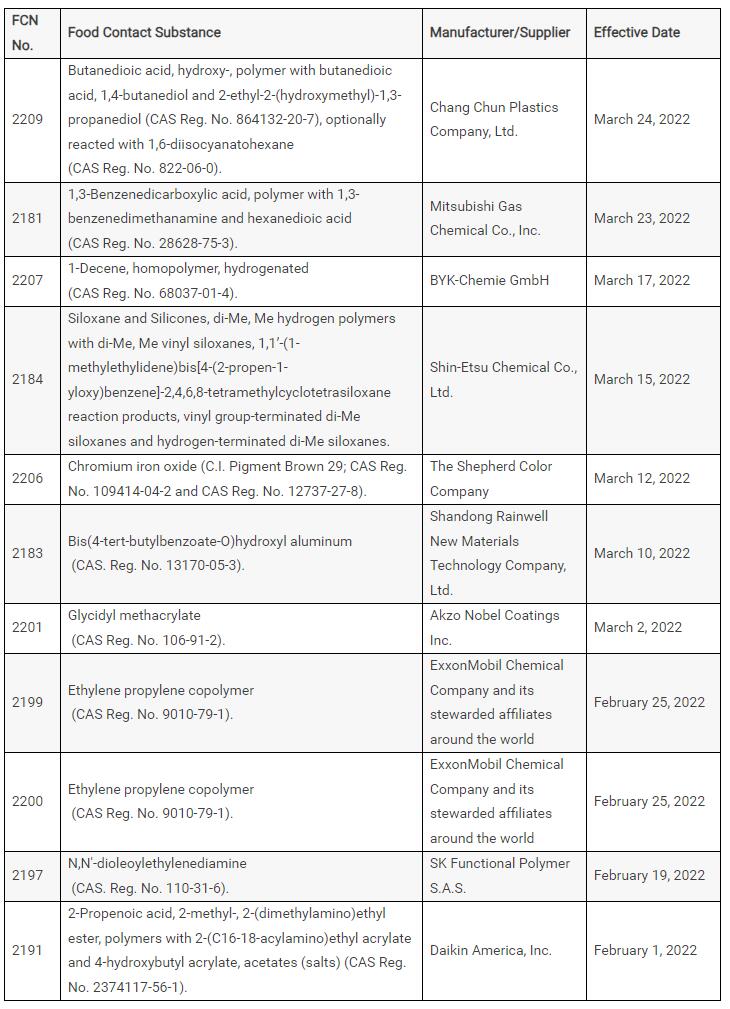
United States FCN declaration
FCN(Food Contact Notifications). In order to ensure food safety, the 1958 regulation required that all food additives (direct and indirect) be approved by the Food and Drug Administration (FDA) before they could be used. For indirect food additives (food contact substances), the FDA has established a food contact notification procedure, which stipulates that substances that are not approved by the FDA and are not prohibited from permitted use need to prepare detailed materials and submit a notice to the FDA, which is called a food Contact Notice (FCN). FCN specificity: The FCN notification in the United States is only valid for the manufacturer/supplier of the substance that has applied, and other manufacturers/suppliers need to declare the same substance to the FDA, and can only use/sell it after the declaration is passed.




 Consultation
Consultation
 400-115-9001
400-115-9001