Cosmetic raw material safety information filling guide - Detailed requirements for raw material submission
"Cosmetic raw material Safety information filling Technical Guidelines (draft for comment)" (hereinafter referred to as the "Guiding Principles (draft for comment)") the rest of the detailed requirements of the key content and the content of raw material safety information update for everyone to sort out and summarize.
Brief description of production process
The production process should be in accordance with the actual production situation, fill in a brief description of the production process of raw materials, focusing on the steps that may be related to the safety risks of raw materials.
In the description of the production process, it should be clear that the type of production process used, such as physical grinding, physical pressing, solvent extraction, chemical synthesis, biological fermentation, etc. A brief overview of the main production steps, such as stirring, heating, distillation, filtration, drying, packaging, etc., may be provided, where specific process parameters are not listed unless they relate to the control of risk substances.
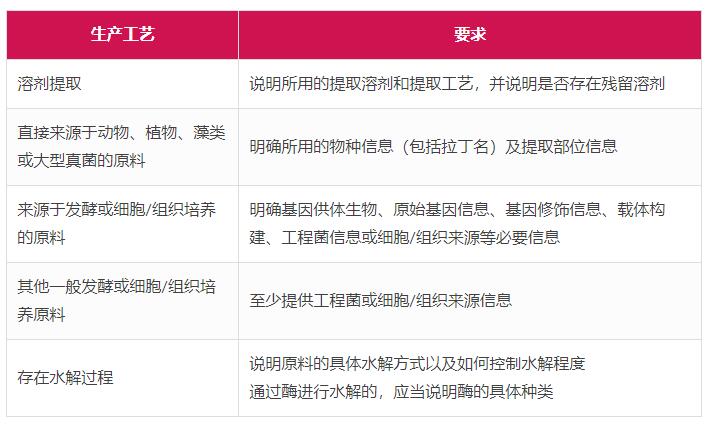
In addition, the Guiding Principles (Draft for Comment) also put forward more detailed requirements for several typical production processes, as shown in the following table.
Quality control requirements
Quality control content
In the process of raw material production, a series of indicators and methods should be set up to effectively control the quality of raw materials. Except for special instructions, cosmetic registrants and recordholders should generally fill in the relevant content of raw material quality control according to the relevant information provided by the raw material manufacturer.
However, the "Guiding Principles (Draft for Comments)" points out that under the premise of agreeing with raw material manufacturers and accepting raw materials to ensure that the relevant raw materials meet the set quality control standards, cosmetics registrants and record holders can also set control indicators or other requirements on their own.
Raw material identification methods
Identification of cosmetic raw materials refers to judging the authenticity of raw materials, which can be identified by the structure and properties of raw materials, chemical reaction, instrumental analysis or determination of physical constants. Cosmetics registrants and record holders may fill in the identification method of raw materials according to the information provided by raw material manufacturers or their own research results.
Control indicators and detection methods
The setting of raw material quality control indicators should be able to reflect the quality standards of raw materials, and the control indicators and detection methods filled in should be consistent with the actual production and quality control of raw materials.
The Guiding Principles (Draft for Comment) put forward detailed requirements for the following types of raw materials:
A single raw material with a clear chemical structure: its purity requirements should be clearly defined.
● Raw materials with unclear chemical structure (such as various animal and plant extracts) : quantitative requirements for indicative components should be provided, or quantitative requirements for total components, evaporation residue/solid content, drying weight loss/moisture, incandescence residue, typical physical and chemical indicators should be provided. If it is used for special effects such as freckle whitening and hair loss prevention, the specific efficacy ingredients and their control standards should generally be specified.
● Polymer raw materials: generally should be clear polymerization degree and average molecular weight.
Oligopeptide raw materials: The amino acid sequence should be clear, and when filling in the sequence, the 20 natural amino acids should use the standard Chinese name, three-letter or single-letter abbreviation.
Nano-raw materials: should be combined with the safety assessment data, at least listed the key parameters affecting the safety assessment of raw materials conclusions.
< International authority assessment conclusion
The "Guiding Principles (Draft for Comment)" lists some common international authorities, such as the European Union Scientific Committee on Consumer Safety (SCCS), the United States Cosmetic Raw Materials Evaluation Committee (CIR), the International Fragrance Association (IFRA), the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO) and so on.
Brief description of requirements for use in other industries
Cosmetics registrants and recordholders may fill in the relevant information about the requirements for the use of raw materials in food, medicine and other industries according to the information provided by raw material manufacturers or their own research results.
Risk substance limit requirements
According to the nature of raw materials, sources, production and processing processes and other relevant information, the possible risk substances in cosmetic raw materials are analyzed, and their limit requirements are clarified.
The Guiding Principles (Draft for Comment) lists common types of risk substances in the document and gives examples, as shown in the following table:

Raw material safety information update
In the last part of the "Guiding Principles (Draft for Comments)" mentioned that cosmetics registrants and recordholders should be in accordance with the "Regulations on the Management of Cosmetics Registration and recordkeeping Data", the updating of raw material safety information should be handled in the following three different situations.
Where there is a change in the product safety assessment data, the product safety assessment data shall also be changed.
● Raw material quality specification information changes, but does not lead to a change in the formula: the raw material safety information needs to be updated and maintained
● Small changes in raw material composition lead to formula changes: product changes
● Material changes: shall not continue to use, cosmetic registrant, recordholder should replace the raw material, and related information update maintenance or change.




 Consultation
Consultation
 400-115-9001
400-115-9001